WONDFO SARS-CoV-2
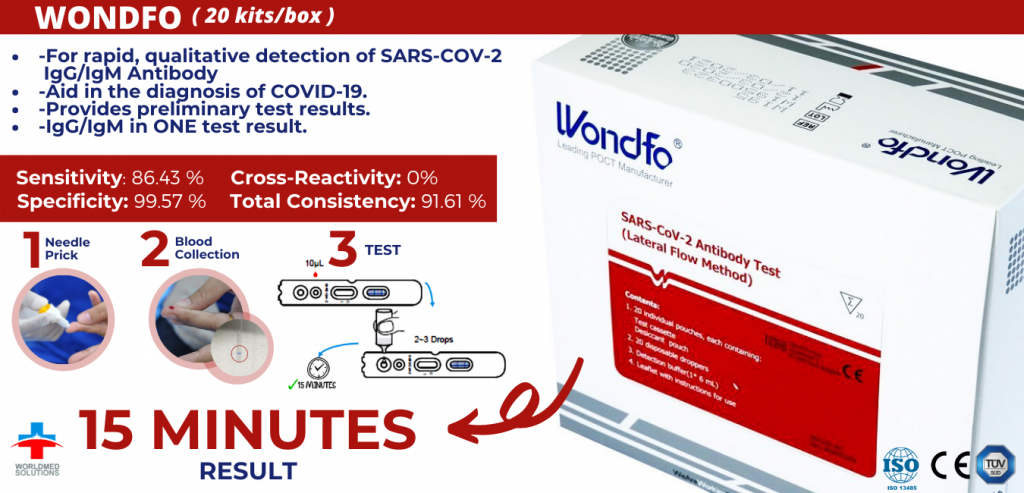
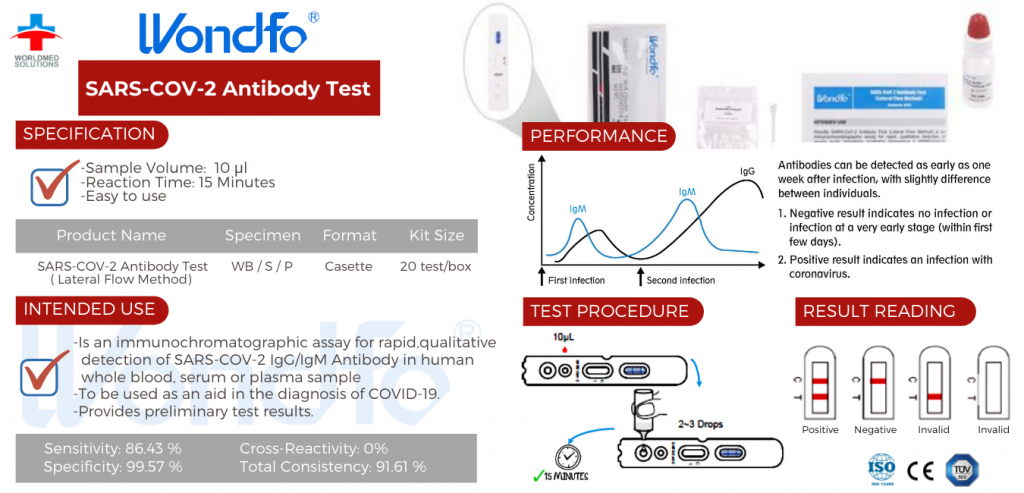
◆ Is an immunochromatographic assay for rapid,qualitative detection of SARs-cov-2 IgG/igM Antibody in human whole blood, serum or plasma sample
◆ To be used as an aid in the diagnosis of COVID-19.
◆ Provides preliminary test results.
**Suitable to detect the IgM and IgG antibodies to severe acute respiratory syndrome corona virus.
- WONDFO is the first officially received certification by the government of China as being accepted for detecting antibodies for COVID-19.
- It is CE, Chinese FDA and Thai FDA Certified.®
Performance:
- Antibodies can be detected as early as one week after infection, with slightly difference between individuals.1. Negative result indicates no infection or infection at a very early stage (within first few days).2. Positive result indicates an infection with coronavirus.
FOR CERTIFICATIONS click below:
– please click here: Certification for Export
– please click here: Certificate of conformity
– please click here: ISO Certification
– please click here: Product Catalog
Wondfo – SARS-CoV-2 Antibody Test (Lateral Flow Method)
✓ Sensitivity: 86.43 %
✓ Specificity: 99.57 %
✓ Cross-Reactivity: 0%
✓ Total Consistency: 91.61 %
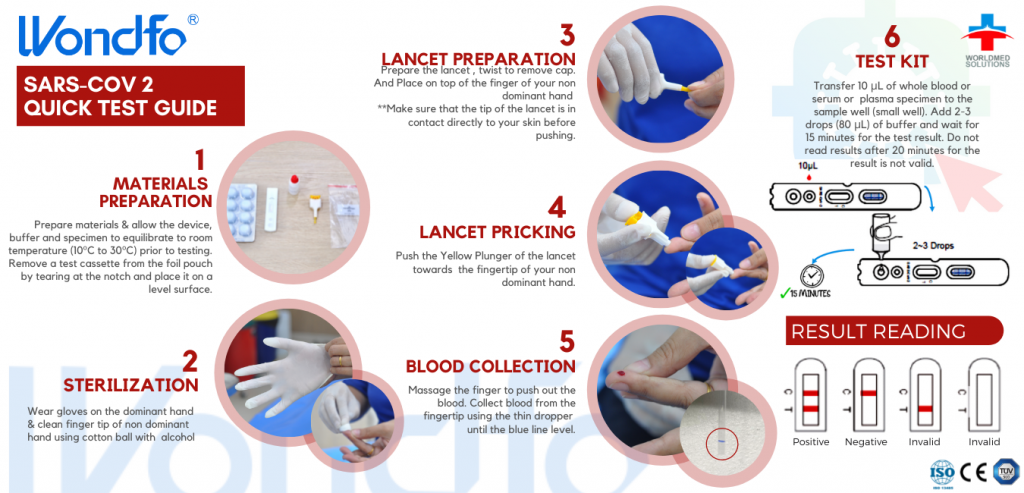
★ Results in 15 Minutes
★ Easy to use.
★ The test provides preliminary test results.
★ Negative results don’t preclude SARS-CoV-2 infection and they cannot be used as the sole basis for treatment or other management decision.